Neuroimaging studies of face perception (PET, fMRI)
Since the pioneering studies of the late Justine Sergent and her colleagues (Sergent et al., 1992, Brain), neuroimaging studies have identified multiple regions in the occipital and temporal lobe, mainly on the ventral occipito-temporal cortex, which respond more to pictures of faces than objects. The role of these regions in face processing, and how they interact with each other remain largely unclear. We first used positron emission tomography (PET) (Dubois et al., 1999; Rossion et al., 2000; Rossion et al., 2001) to clarify these questions in the normal human brain, and then fMRI, from our first study (Rossion et al., 2003) up to most recent studies (Jiang et al., 2015; Jonas et al., 2015).
Our research has concentrated on several aspects of the neural basis of face perception:
* The contrast between unfamiliar and familiar faces, which has been a topic of interest since the earliest PET studies (Dubois et al., 1999; Rossion et al., 2000; Rossion et al., 2003) until our most recent (fMRI) work with gradual revealing of visual information in fMRI (Ramon et al., in press). For instance, we found that areas of the right ventral occipito-temporal cortex repond in a categorical way to face familiarity rather than according to the degree of similarity to a familiar face (Rossion et al., 2001). We found these effects also in the so-called fusiform face area (FFA) and occipital face area (OFA), that we localized in PET using a faces vs. objects localizer approach (Rossion et al., 2003). We came back to this initial interest for morphed faces and familiarity in fMRI (Ramon et al., 2010).
* The first demonstration that face-selective areas, at least the right FFA, and to a lesser extent, the left FFA and the OFAs, represent individual faces holistically (i.e., as an integrated representation rather than as separated facial parts). Again, our early studies using PET provided evidence for this holistic representation in the right FFA (Rossion et al., 2000). Our subsequent fMRI studies using the well-know composite face illusion and fMRI-adaptation (Schiltz & Rossion; 2006; Schiltz et al., 2010) have demonstrated the holistic representation of individual faces in the right middle fusiform gyrus.
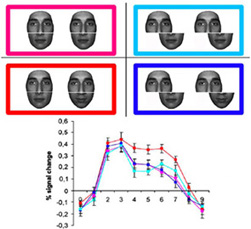
* The definition of the set of brain areas responding preferentially to faces than controlled objects in a large-scale fMRI study (Rossion et al., 2012) and how the relative lateralization is affected by handedness. While the right FFA is larger in right-handers, the left FFA is at least as large or even larger than the right in left-handers (Bukowski et al., 2013). However, other face-selective areas of the network remain larger in the right hemisphere even in left handers.
* The proposal of a non-hierarchical view of selective processing of faces in the ventral occipito-temporal cortex (Rossion et al., 2003; Rossion, 2008), suggesting that the FFA can be recruited independently/before the OFA. This proposal was initially based on fMRI studies of brain-damaged patients presenting impairments of face recognition (prosopagnosia) and the observation of face-selectivity in the right middle fusiform gyrus (‘FFA’) in prosopagnosic patient PS, despite an extensive lesion of the inferior occipital cortex, posteriorly located (Rossion et al., 2003 ). This findings has important implications for neuro-anatomical models of face perception because they cast doubts on the hypothesis that the FFA depends on inputs from posterior face-selective regions. See the slideshow of this original paper. See also identical findings in another brain-damaged case by Steeves et al. (2006, Neuropsychologia).
Subsequently, we showed that the 'FFA' of the prosopagnosic patient PS shows identical responses to same or different identities of faces, in line with the behavior of the patient (see Schiltz et al., 2006; Dricot et al., 2008; similar findings for patient DF by Steeves et al., 2009).
This combination of neuroimaging studies of patients with prosopagnosia has inspired other studies to demonstrate this non-hierarchical view of the cortical processing of faces, using Mooney and Acrimboldo stimuli (Rossion et al., 2011) or gradual revealing of faces in visual scenes (Jiang et al., 2012; Jiang et al., 2015).
* The combination of fMRI with intracerebral elecrophysiological recordings in rare cases of transiently evoked prosopagnosia (Jonas et al., 2012; Jonas et al., 2014). Our most recent work reveals that an area of the right anterior fusiform gyrus, anterior to the FFA, is critical for face recognition, but has been missed by fMRI studies because it lies exactly at the location of the largest signal drop-out due to a magnetic susceptibility artefact (Jonas et al., 2015).
Related Papers (1999-2015)
| PET | Dubois, S., Rossion, B., Schiltz, C., Bodart, J.M., Michel, C., Bruyer, R., Crommelinck, M. (1999). Effect of familiarity on the processing of human faces. Neuroimage, 9, 278-289. [PDF] | |
| PET | Rossion, B., de Gelder, B., Dricot, L., Zoontjes, R., De Volder, A., Bodart, J.-M., Crommelinck, M. (2000). Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus, Journal of Cognitive Neuroscience, 12, 793-802. [PDF] [slideshow summary] | |
| PET | Rossion, B., Schiltz, C., Robaye, R., Pirenne, D., Crommelinck, M. (2001). How does the brain discriminate familiar and unfamiliar faces: a PET study of face categorical perception. Journal of Cognitive Neuroscience, 13, 1019-1034. [PDF] | |
| PET | Rossion, B., Schiltz, C., Crommelinck, M. (2003). The functionally defined ‘face areas’ are sensitive to long-term visual familiarity. NeuroImage, 19, 877-883. [PDF] | |
|
fMRI Prosopagnosia |
Rossion, B., Caldara, R., Seghier, M., Schuller, A.-M., Lazeyras, F., Mayer, E. (2003). A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain Journal of Neurology, 126, 2381-2395. [PDF] [slideshow summary] | |
| fMRI | Mazard, A., Schiltz, C ., Rossion, B. (2006). Recovery from adaptation to facial identity is larger for upright than inverted faces in the human occipito-temporal cortex. Neuropsychologia, 44, 912-922. [PDF] [slideshow summary] | |
|
fMRI Prosopagnosia |
Schiltz C, Sorger B, Caldara R, Ahmed F, Mayer E, Goebel R, Rossion B. (2006). Impaired face discrimination in acquired prosopagnosia is associated with abnormal response to individual faces in the right middle fusiform gyrus. Cerebral Cortex, 16, 574-586. [PDF] [slideshow summary] | |
|
fMRI Prosopagnosia |
Sorger, B., Goebel, R., Schiltz, C., Rossion, B. (2007). Understanding the functional neuroanatomy of prosopagnosia . NeuroImage, 35, 836 - 852. [PDF] | |
|
fMRI Prosopagnosia |
Dricot, L., Sorger, B., Schiltz, C., Goebel, R., Rossion, B. (2008). The roles of “face” and “non-face” areas during individual face perception: evidence by fMRI adaptation in a brain-damaged prosopagnosic patient. NeuroImage, 40, 318-332. [PDF] | |
| REVIEW | Rossion, B. (2008). Constraining the cortical face network by neuroimaging studies of acquired prosopagnosia. NeuroImage, 40, 423-426. [PDF] | |
| fMRI |
Jiang, F., Dricot, L., Blanz, V., Goebel, R., Rossion, B. (2009). Neural correlates of shape and surface reflectance information in individual faces. Neuroscience, 163, 1078-1091. [PDF] |
|
|
fMRI Prosopagnosia |
Steeves, J., Dricot, L., Goltz, H., Sorger, B., Peters, J., Milner, D., Goodale, M.-A., Goebel, R., Rossion, B. (2009). Abnormal face identity coding in the middle fusiform gyrus of two brain-damaged prosopagnosic patients. Neuropsychologia, 47, 2584-2592. [PDF] |
|
|
fMRI
|
Schiltz, C., Dricot, L., Goebel, R., & Rossion, B. (2010). Holistic perception of individual faces in the right middle fusiform gyrus as evidenced by the composite face illusion. Journal of Vision, 10(2):25, 1-16. [PDF] |
|
|
fMRI
|
Ramon, M., Dricot, L., Rossion, B. (2010). Personally familiar faces are perceived categorically in face-selective regions other than the FFA. European Journal of Neuroscience, 32, 1587-1598. [PDF] |
|
| fMRI |
Jiang, F., Dricot, L., Weber, J., Righi, G., Tarr, M.J., Goebel, R., Rossion, B. (2011). Face categorization in visual scenes may start in a higher order area of the right fusiform gyrus: evidence from dynamic visual stimulation in neuroimaging. Journal of Neurophysiology, 106, 2720-2736. [PDF] [stimuli as figures] [video] |
|
|
fMRI
|
Rossion, B., Dricot, L., Goebel, R., Busigny, T. (2011). Holistic face categorization in higher-level cortical visual areas of the normal and prosopagnosic brain: towards a non-hierarchical view of face perception. Frontiers in Human Neuroscience, 4:225. doi: 10.3389/fnhum.2010.00225. [PDF] |
|
| fMRI |
Rossion, B., Hanseeuw, B., Dricot, L. (2012). Defining face perception areas in the human brain: a large-scale factorial fMRI face localizer analysis. Brain and Cognition, 79, 138-157. [PDF] |
|
| fMRI Prosopagnosia iEEG |
Jonas, J., Descoins, M., Koessler, L., Colnat-Coulbois, S., Sauvee, M., Guye, M., Vignal, J-P., Vespignani, H., Rossion, B., Maillard, L. (2012). Focal electrical intracerebral stimulation of a face-sensitive area causes transient prosopagnosia. Neuroscience, 222, 281-288. [PDF] [video 1] [video 2] | |
|
fMRI
|
Bukowski, H., Dricot, L., Hanseeuw, B., & Rossion, B. (2013). Cerebral lateralization of face-sensitive areas in left-handers: only the FFA does not get in right. Cortex, 49, 2853-2859. [PDF] |
|
| REVIEW | Rossion, B. (2014). Understanding face perception by means of prosopagnosia and neuroimaging. Frontiers in Bioscience (Elite Ed.); 6-308-317. [PDF] | |
| fMRI Prosopagnosia iEEG |
Jonas, J., Rossion, B., Krieg, J., Koessler, L., Colnat-Coulbois, S., Maillard, L., Frismand, S., Colnat-Coulbois, S., Vignal, J.-P., Vespignani, H., Jacques, C., Brissart, H., Maillard, L. (2014). Intracerebral electrical stimulation of a face-selective area in the right inferior occipital cortex impairs individual face discrimination. NeuroImage, 99, 487-497. [PDF] [videos] | |
| fMRI | Gentile, F., & Rossion, B. (2014). Temporal frequency tuning of cortical face-sensitive areas for individual face perception. NeuroImage, 90, 256-265. [PDF] | |
| fMRI |
Jiang, F., Badler, J., Righi, G., Rossion, B. (2015). Category search speeds up face-selective fMRI responses in a non-hierarchical cortical face network. Cortex, 66, 69-80.[PDF] |
|
| fMRI Prosopagnosia iEEG |
Jonas, J., Rossion, B., Brissart, H., Frismand, S., Jacques, C., Hossu, G., Colnat-Coulbois, S., Vespignani, Vignal, Maillard, L. (in press). Beyond the core face-processing network: intracerebral stimulation of a face-slective area in the right anterior fusiform gyrus elicits transient prosopagnosia. Cortex, in press. [PDF] [VIDEOS] |


